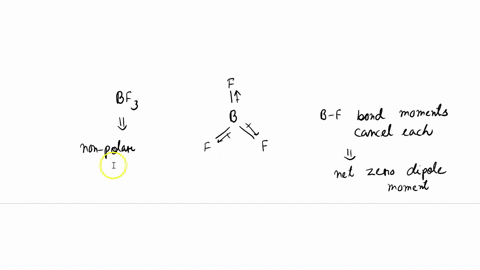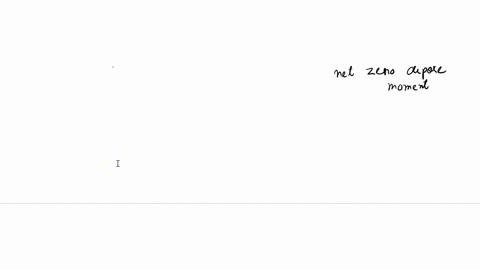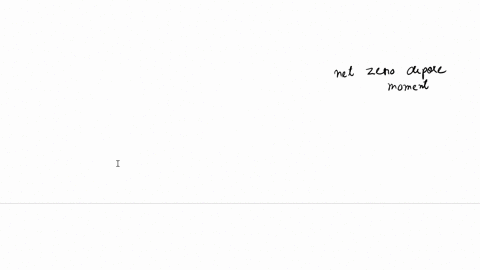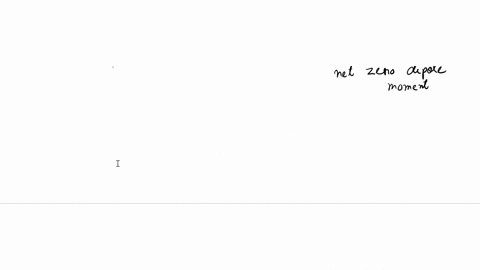
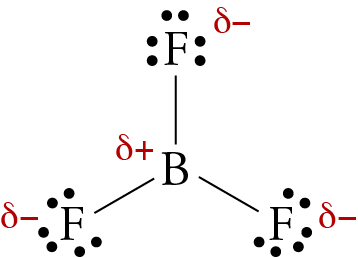
BF3 Molecular Geometry, Shape and Bond Angles (Boron Trifluoride) | BF3 Molecular Geometry, Shape and Bond Angles (Boron Trifluoride) Today in this video, we help you determine the molecular geometry of a

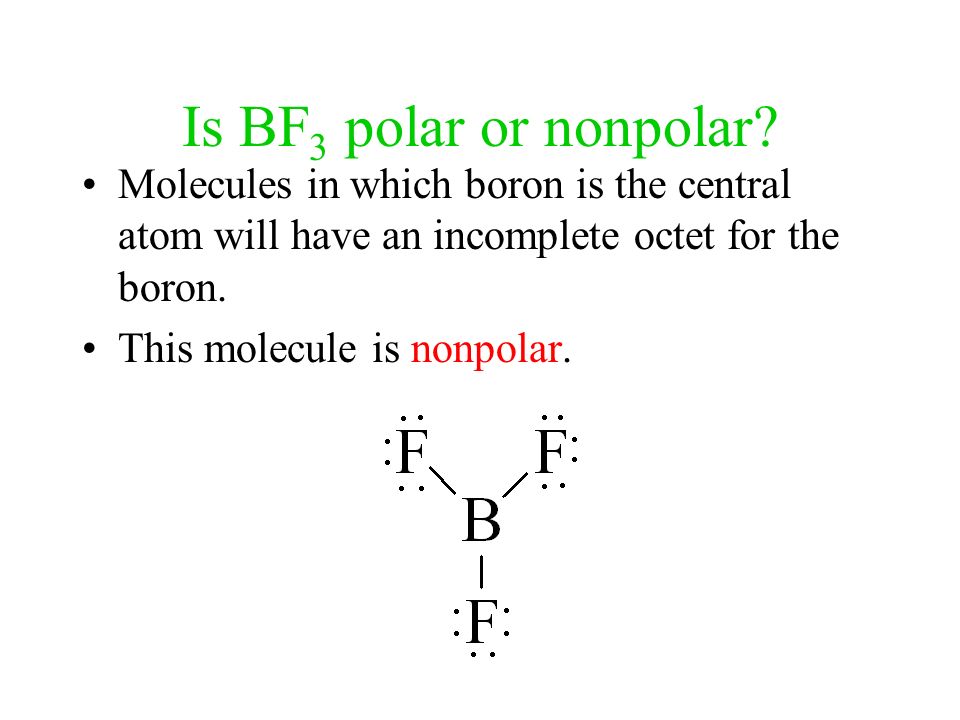
1 POLARITY POLAR BONDS Bonds between atoms POLAR MOLECULES Polarity between molecules Occurs when polar bonds create a dipole moment. - ppt download
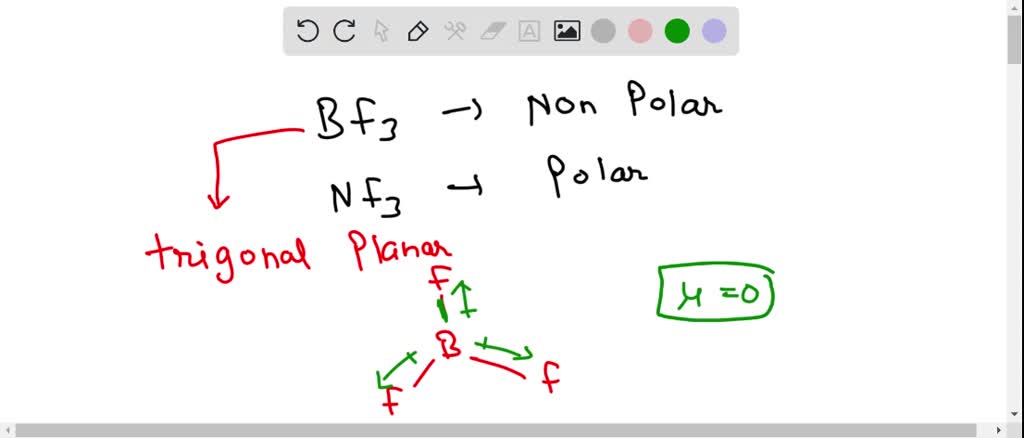
SOLVED: 41. The BF3 molecule is nonpolar, whereas the NF3 molecule is polar. Which of the following statements accounts for the difference in polarity of the two molecules? In NF3, each F
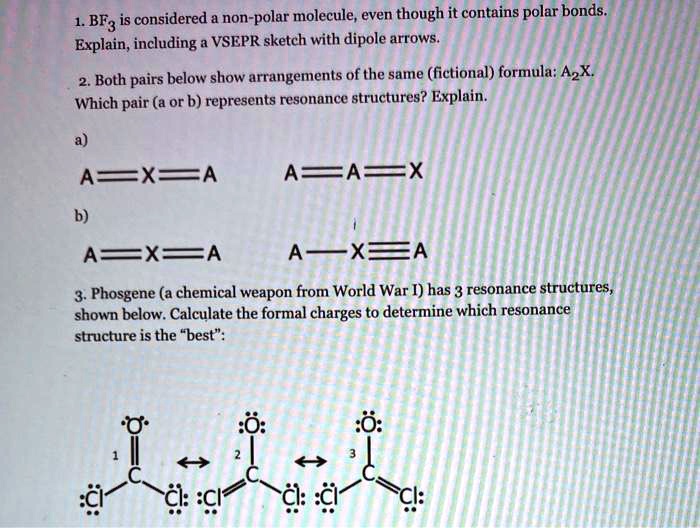
SOLVED: BF3 is considered a nonpolar molecule, even though it contains polar bonds. Explain, including a VSEPR sketch with dipole arrows. Both pairs below show arrangements of the same (fictional) formula: AzX.
Boron trifluoride (displaystyle B{ F }_{ 3 }) is a nonpolar molecule, whereas ammonia (displaystyle N{ H }_{ 3 }) is a polar molecule. The difference in polarities is related to the