PO43- Lewis structure, molecular geometry or shape, bond angle, hybridization, polar vs non-polar | Molecular geometry, Molecular, Vsepr theory
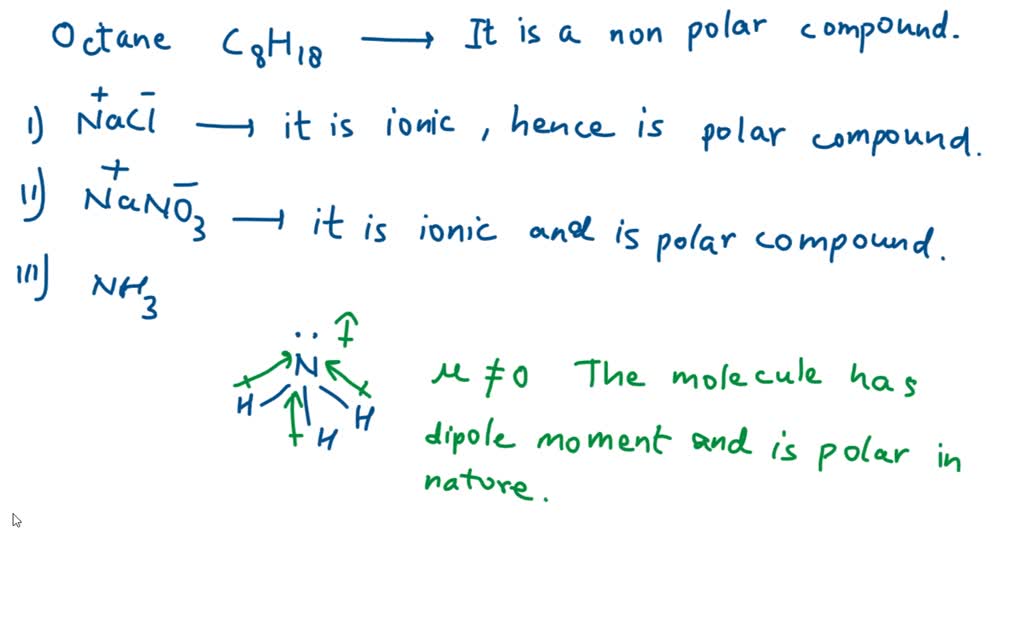
SOLVED: 37. Which of the following should be most soluble in octane (C8H18)? NaCl, NaNO3, NH3, CH3OH, Br2.
Molecular structure of type II kerogen fragment (left), nonpolar oil... | Download Scientific Diagram

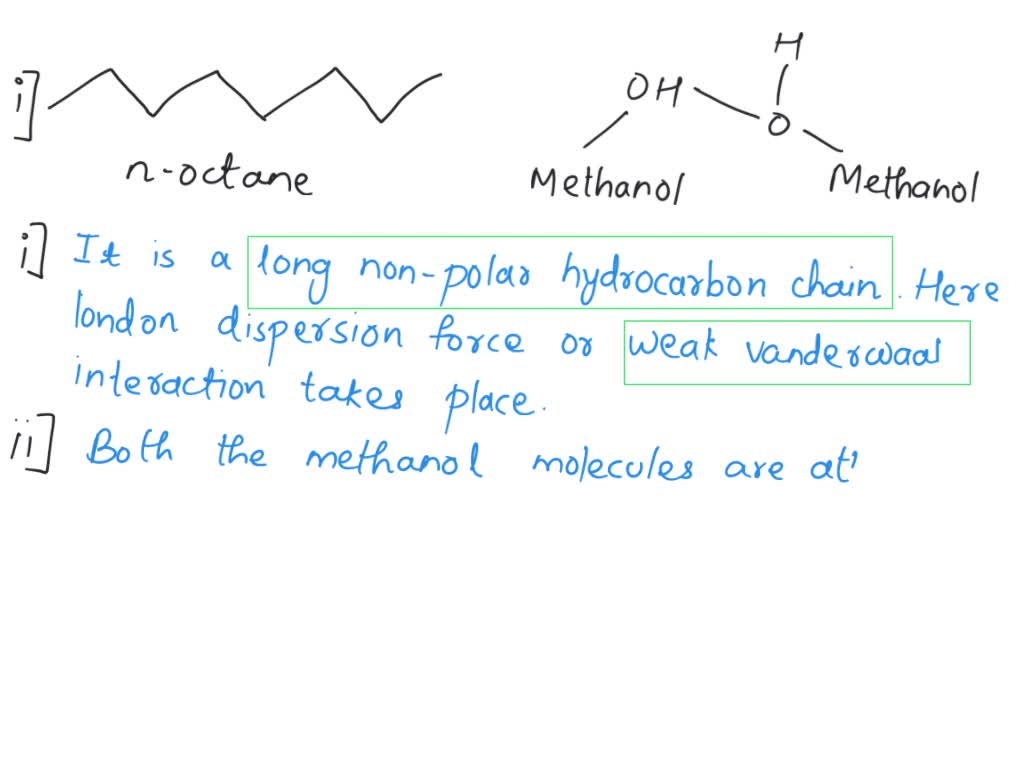
ch 13chem.pdf - Chapter 13 1 The dissolution of water in octane C8H18 is prevented by . A London dispersion forces between octane molecules B | Course Hero
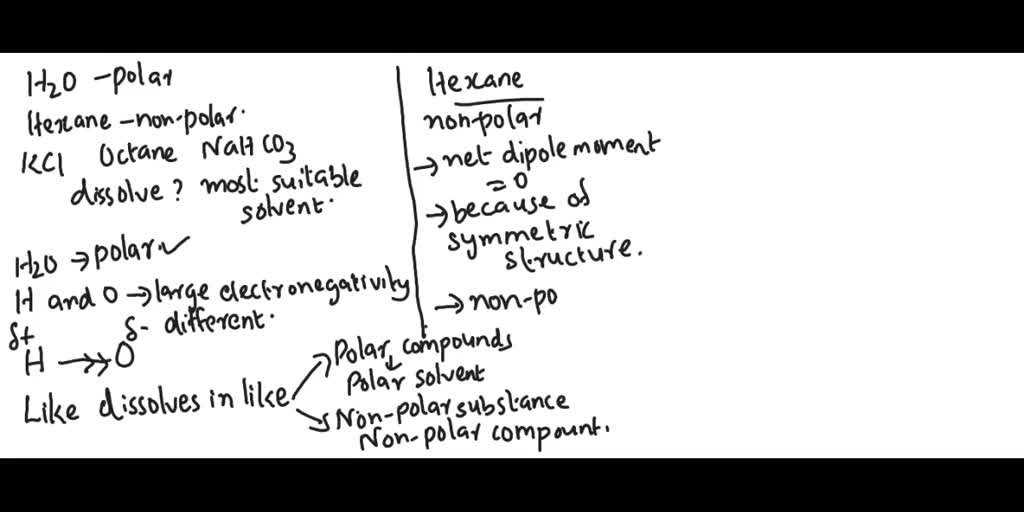
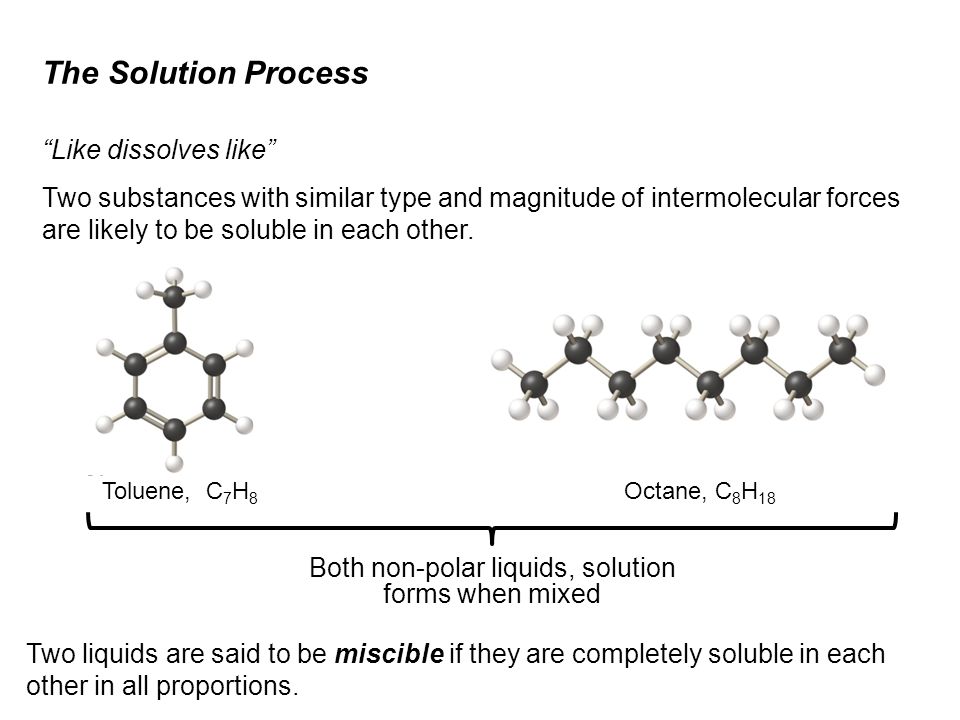
SOLVED: Water is a polar solvent and hexane is a non-polar solvent. Determine which solvent each of the following is most likely to be soluble in. Potassium chloride, KCl Octane, C8H18, a

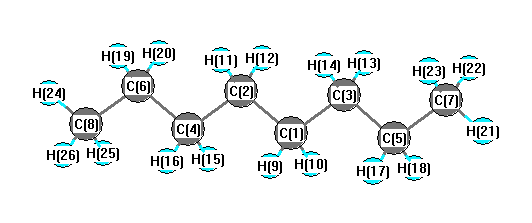
a) Are there polar bonds in Hexane? (Show EN for each of the bonds in the molecule) b) What is the geometric shape of Hexane (VSEPR)? c) Is this molecule polar or