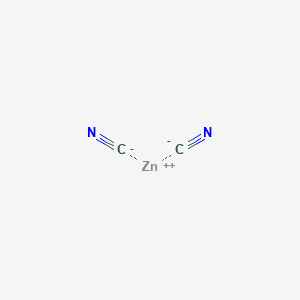
SOLVED: Table 2. Complex Ions and Dissociation Constants Complex Name Formula K Value Tetraamminecadmium (II) Tetraamminecopper (II) Diamminesilver (I) Tetraamminezinc Hexaamminenickel (II) Tetrahydroxoaluminate Tetrahydroxochlorate (III ...

IONIC BONDING. What is an ion? An ion: an atom or bonded group of atoms with a positive or negative charge Cation: A positively charged ion Anion: A negatively. - ppt download

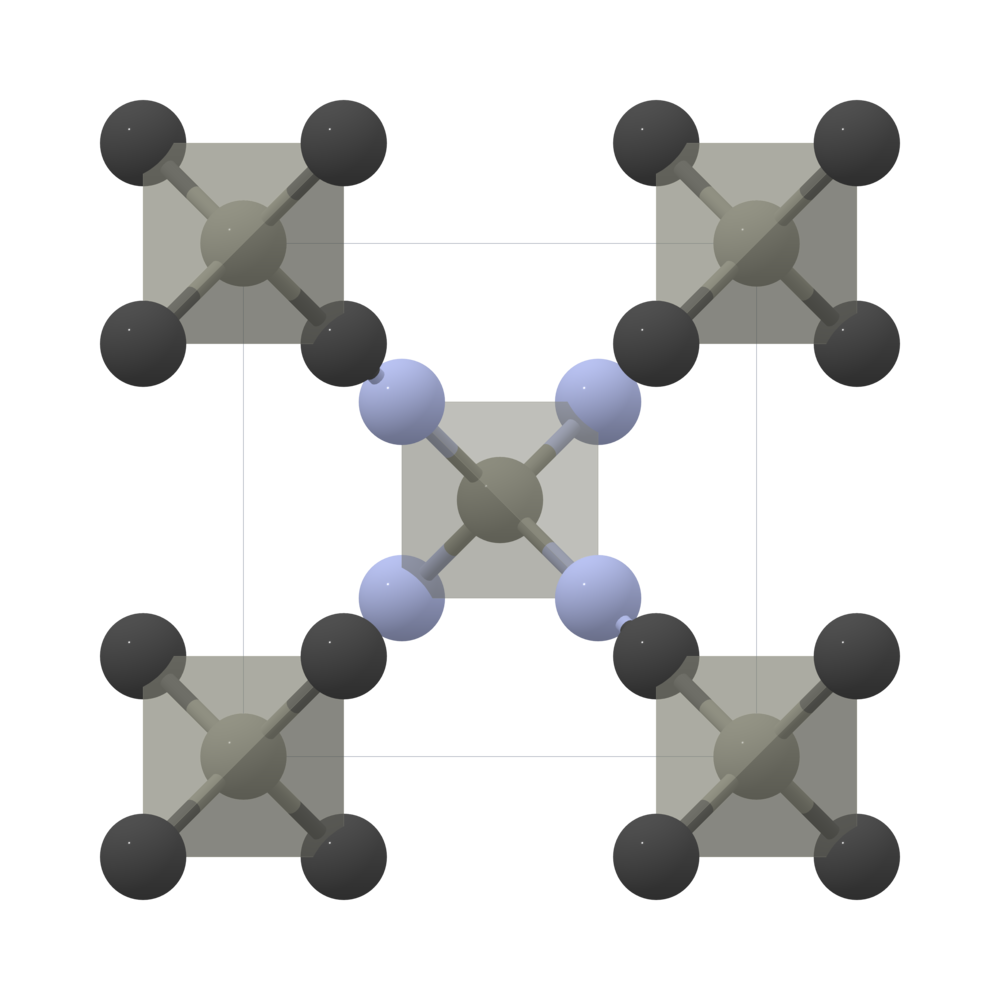
Crystal structure of Zn(CN) 2 showing the two sublattices where one is... | Download Scientific Diagram

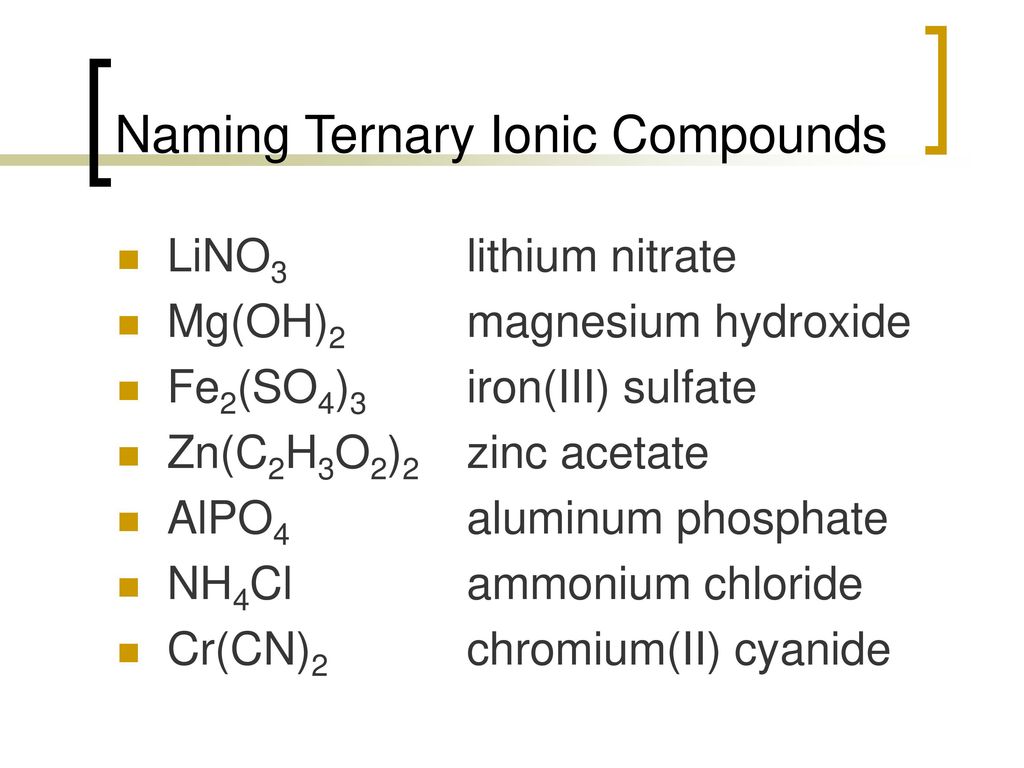
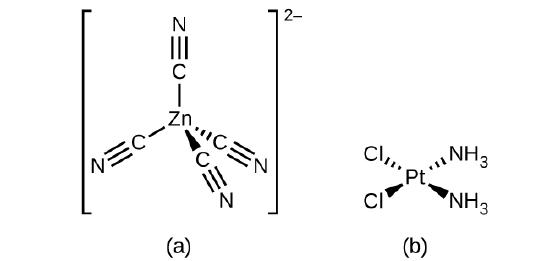
Write formulas for the following compounds. (a) trans-diamminedinitroplatinum(II) (b) rubidium tetracyanozincate (c) triaqua-cis-dibromochlorochromium(III) (d) pentacarbonyliron(0) | Homework.Study.com

Zinc cyanide manufacturers, suppliers, exporters, producers, in Visakhapatnam India. | Vizag Chemicals
![Polymorphism of Zn[Au(CN)2]2 and Its Luminescent Sensory Response to NH3 Vapor | Journal of the American Chemical Society Polymorphism of Zn[Au(CN)2]2 and Its Luminescent Sensory Response to NH3 Vapor | Journal of the American Chemical Society](https://pubs.acs.org/cms/10.1021/ja801773p/asset/images/large/ja-2008-01773p_0012.jpeg)
Polymorphism of Zn[Au(CN)2]2 and Its Luminescent Sensory Response to NH3 Vapor | Journal of the American Chemical Society
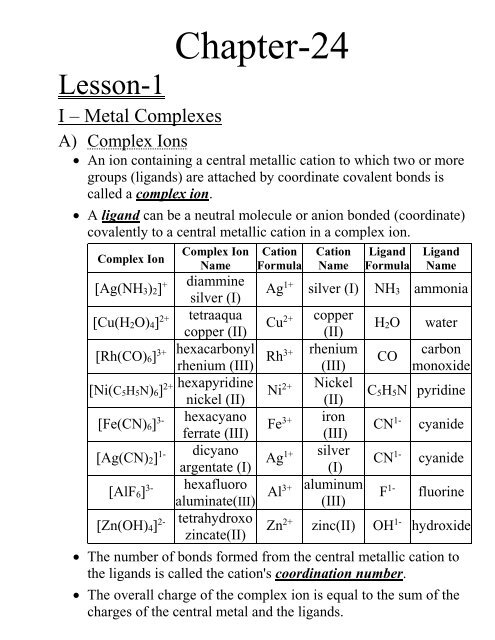
![SOLVED: a) Name the following complex compounds: i) [CoCl2(NH3)4] ii) [Pt(H2O)2(CN)2] iii) [CoCl2(en)2] iv) K4[Fe(CN)6] v) [PtCl4]2- vi) [Ni(OH)2(H2O)3] vii) [Zn(en)3]2+ viii) [CoCl6]3- b) In complex [CoCl2(NH3)4], what is the valence of SOLVED: a) Name the following complex compounds: i) [CoCl2(NH3)4] ii) [Pt(H2O)2(CN)2] iii) [CoCl2(en)2] iv) K4[Fe(CN)6] v) [PtCl4]2- vi) [Ni(OH)2(H2O)3] vii) [Zn(en)3]2+ viii) [CoCl6]3- b) In complex [CoCl2(NH3)4], what is the valence of](https://cdn.numerade.com/ask_images/da9de62ce76c4ba8a5272d89467b69a4.jpg)
![What is the name of this compound : Na2[Zn(CN)4]. ? - Brainly.in What is the name of this compound : Na2[Zn(CN)4]. ? - Brainly.in](https://hi-static.z-dn.net/files/dcd/389ca146ac1efd0881c78272eb2b8e9e.jpg)


![Write the IUPAC name of (i)Zn(2)[Fe(CN)(6)] (ii) Pt[Cl(2)(NH(3))(2) Write the IUPAC name of (i)Zn(2)[Fe(CN)(6)] (ii) Pt[Cl(2)(NH(3))(2)](https://static.doubtnut.com/ss/web/14786220.webp)




![The complex K(4)[Zn(CN)(4)(O(2))(2)] is oxidised into K(2)[Zn(CN)(4)(O The complex K(4)[Zn(CN)(4)(O(2))(2)] is oxidised into K(2)[Zn(CN)(4)(O](https://static.doubtnut.com/ss/web-overlay-thumb/1263761.webp)