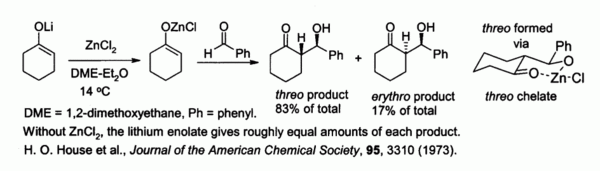
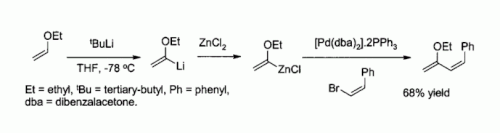
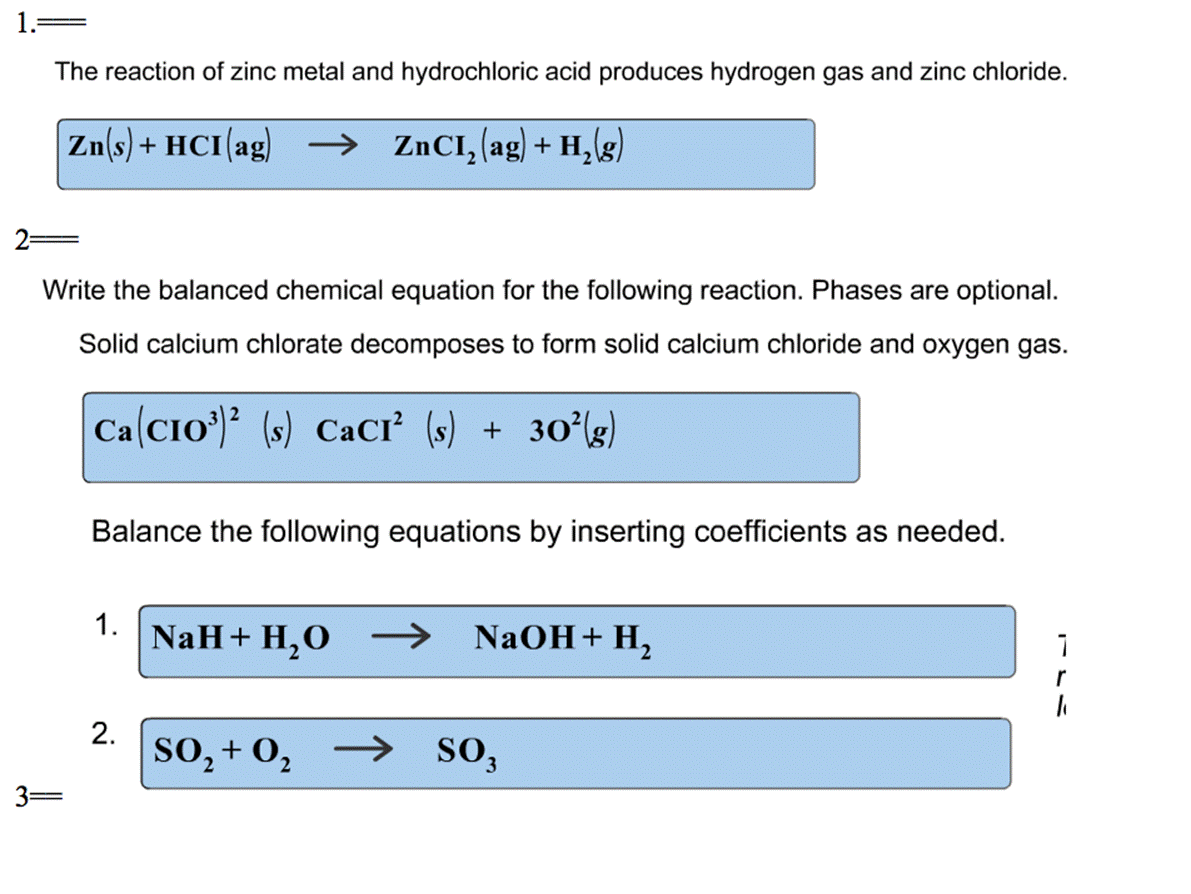
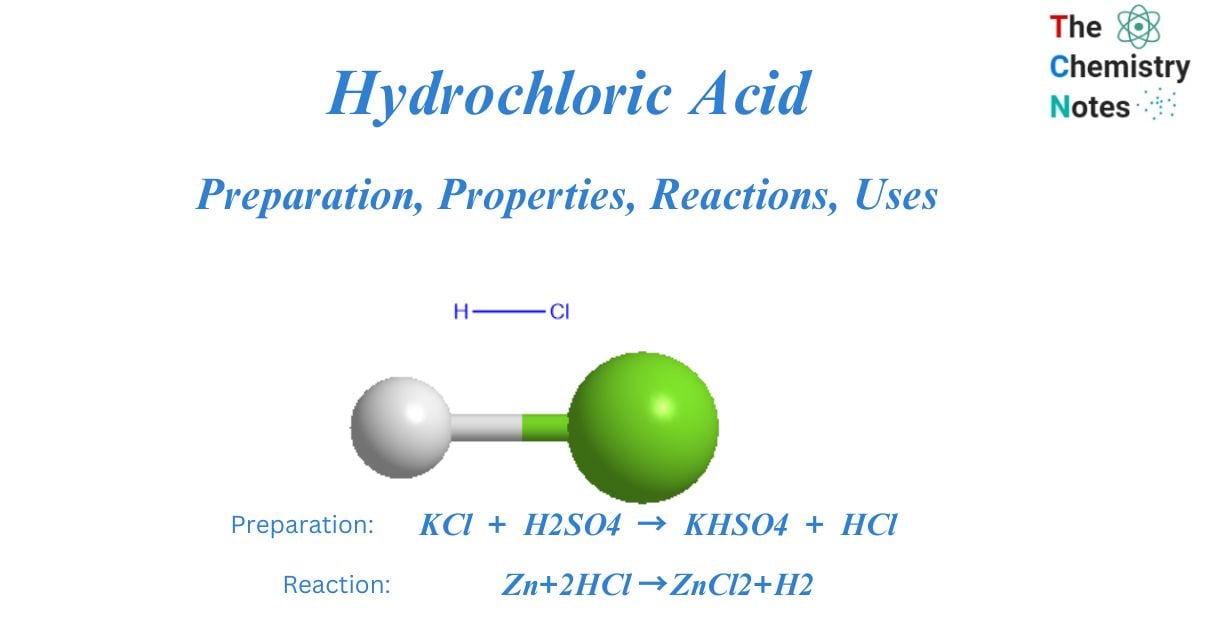
In the chemical equation Zn + 2HCL → ZnCl2 + H2, the reactants are A. zinc chloride and hydrogen. B. zinc - brainly.com

28. Columni Column II (p) 50% of excess reagent left (A) Zn(s) + 2HCl(aq) → ZnCl (s) + H2(g) above reaction is carried out by taking 2 moles each of Zn and
If 20.0 grams of zinc react with excess hydrochloric acid, how many grams of zinc chloride are produced? Zn + HCl = ZnCl2 + H2? - Quora